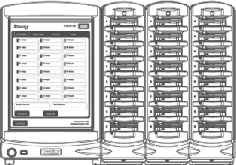
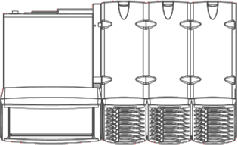
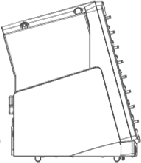
The eSensor® XT-8 is an FDA cleared in-vitro DNA diagnostics instrument currently
produced by Osmetech Molecular Diagnostics. Development of the XT-8 instrument was
a multi-million dollar project involving a half-dozen contractors responsible for
software, hardware, circuitry, firmware, graphic design, industrial design and manufacturing.
I was directly involved in all aspects of the instrument development, project scheduling,
costing, testing and validation. I managed the external contractors, internal employees,
ensured that design controls were followed and authored the software sections of
the FDA 510(k) submission.